Ethylene glycol is a synthetic liquid substance that absorbs water. It is odorless, but has a sweet taste. Ethylene glycol is used to make antifreeze and de-icing solutions for cars, airplanes, and boats. It is also used in hydraulic brake fluids and inks used in stamp pads, ballpoint pens, and print shops.. 15.999. 31.998. Total Mass. 62.068. The molar mass of C2H6O2 (ethylene glycol) is: 62.068 grams/mol. See also our theoretical yield calculator for chemical reactions (probably your next stop to finish the problem set).
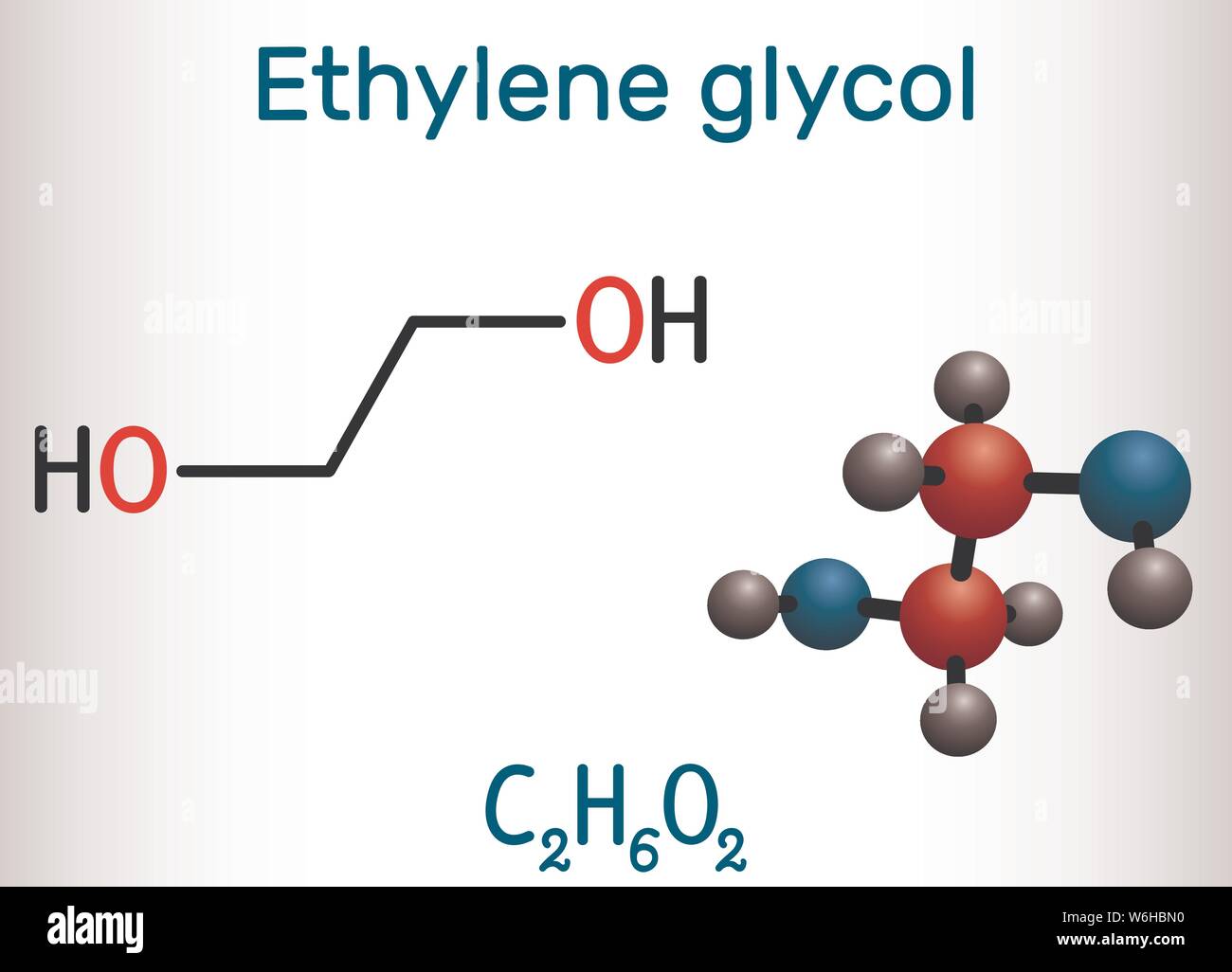
Ethylene Glycol Structural Formula

45g of ethylene glycol (C2H6O2) is mixed with 600g of water. Calculate (a) the freezing point

What is the mass ratio of ethylene glycol (C2 H6 O2 , molar mass =62 g/mo..

Answered What is the mass of ethylene glycol… bartleby

Calculate the mole fraction of ethylene glycol in a solution containing 20 of C2H6O2 by mass

What mass of ethylene glycol (Molar mass = 62.0 g mol^1 ) must be added to 5.50 kg of water to
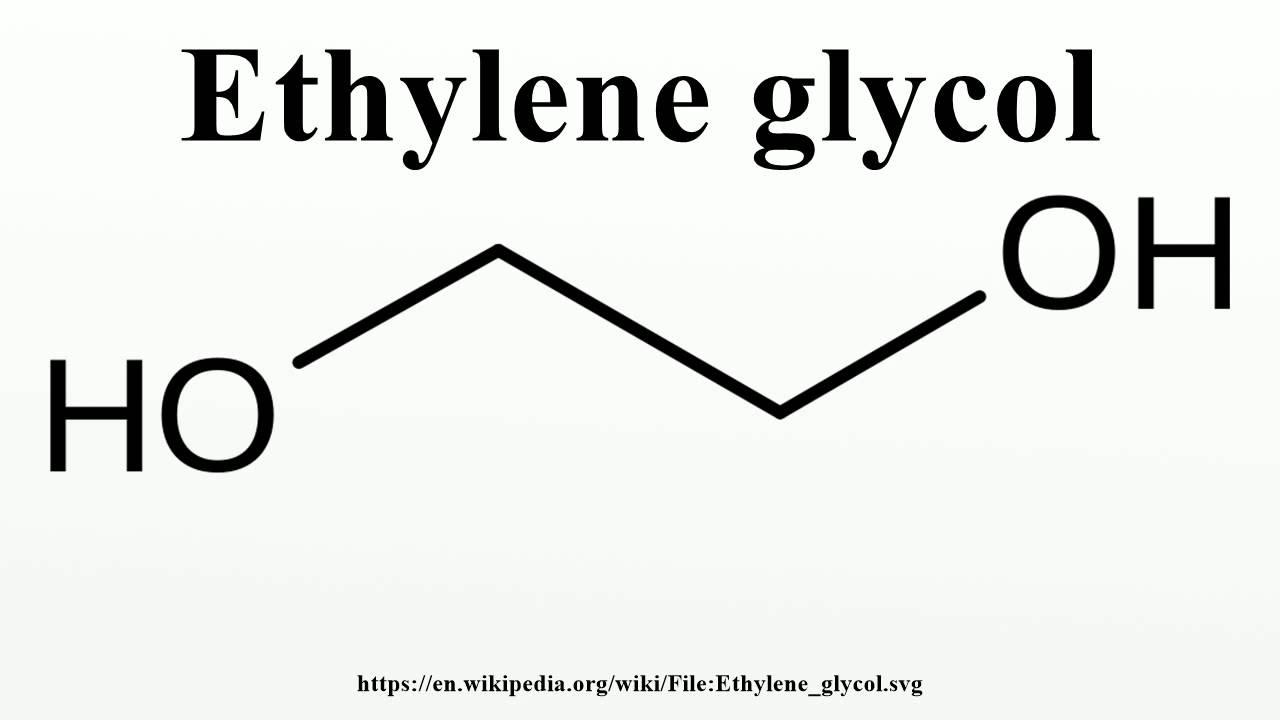
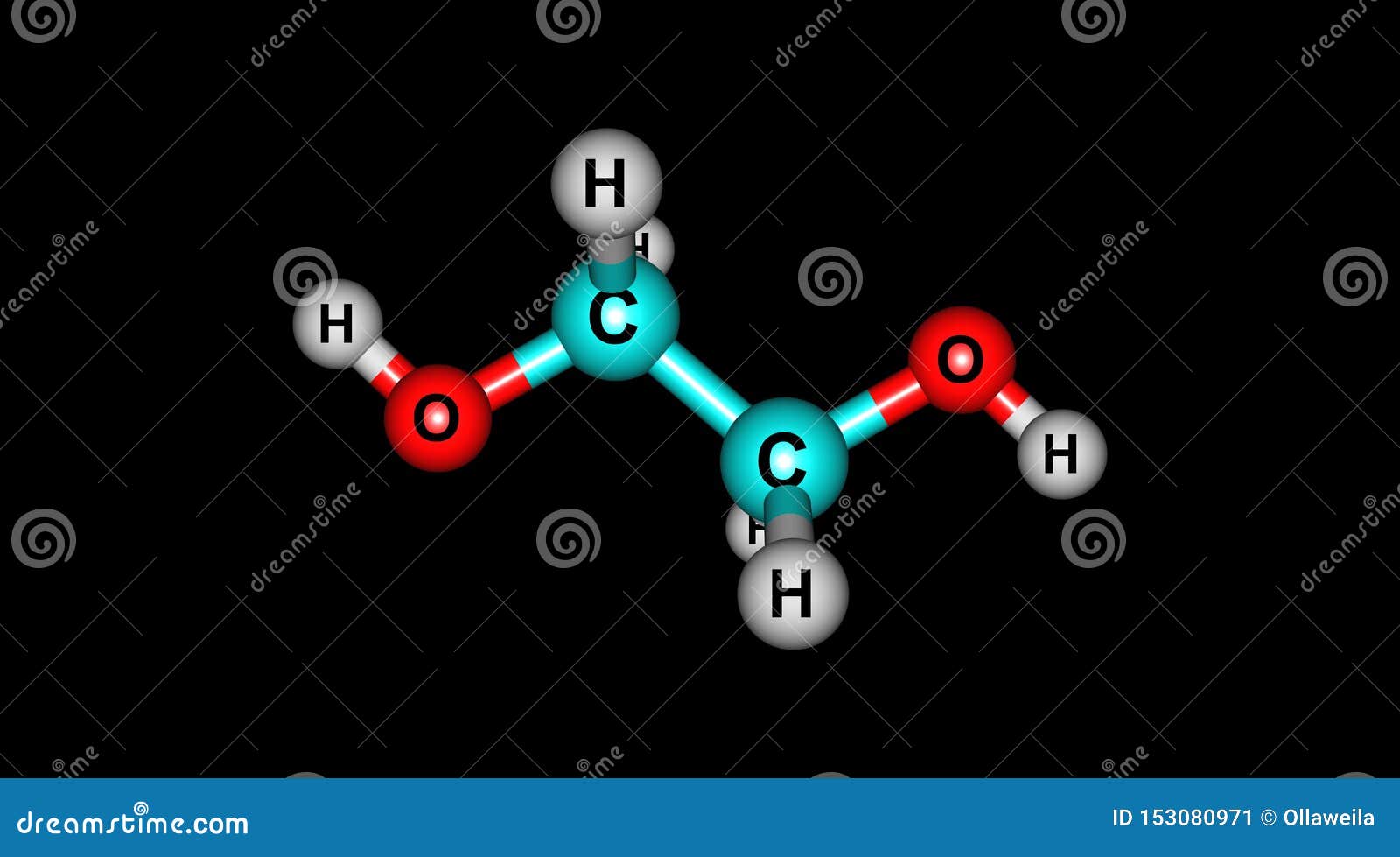
Ethylene Glycol Structure
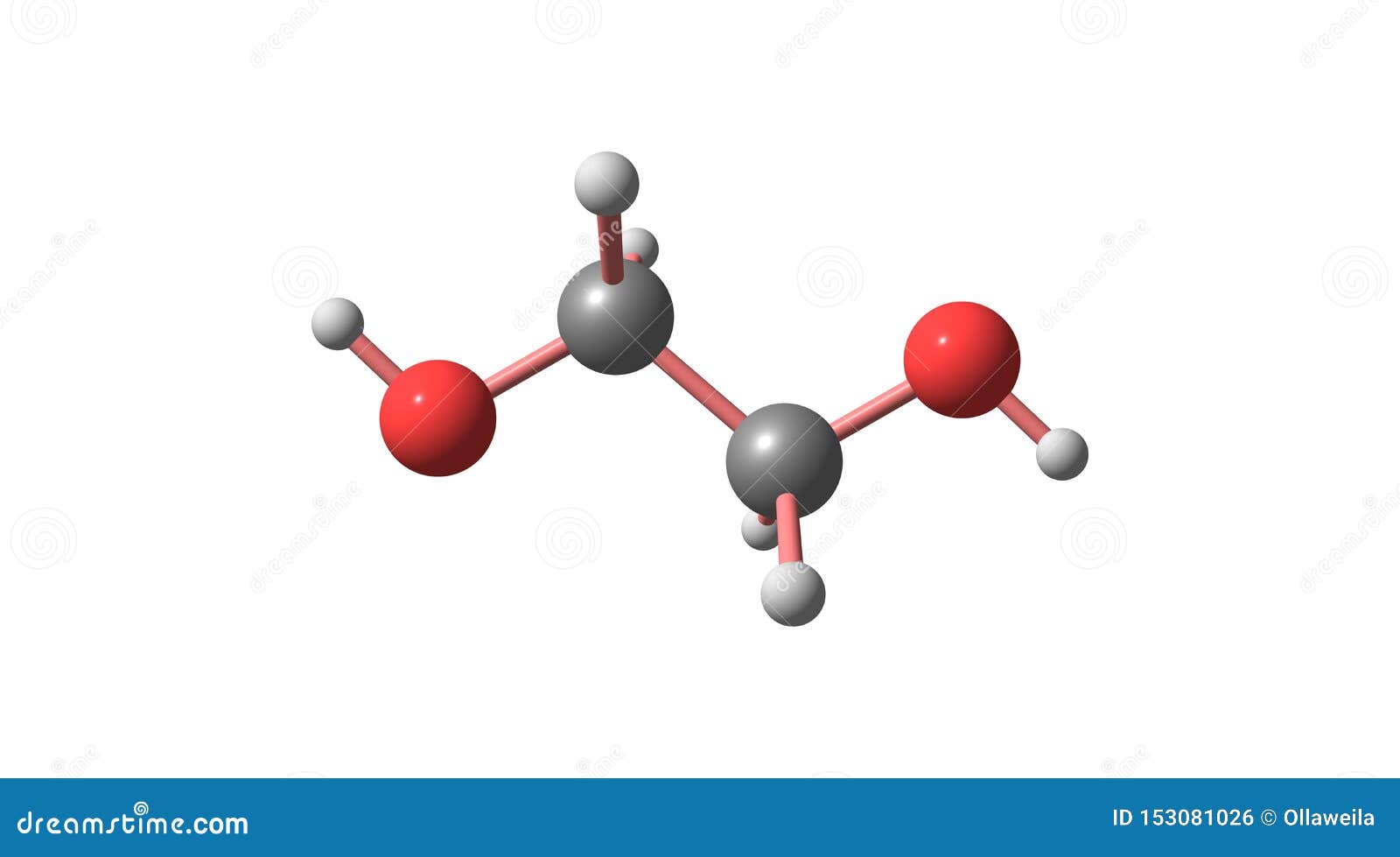
Ethylene Glycol Molecular Structure Isolated on White Stock Illustration Illustration of

Methylene Glycol (CH2(OH)2) Molecular Weight Calculation Laboratory Notes

Difference Between Ethylene Glycol and Polyethylene Glycol Definition, Properties, Uses
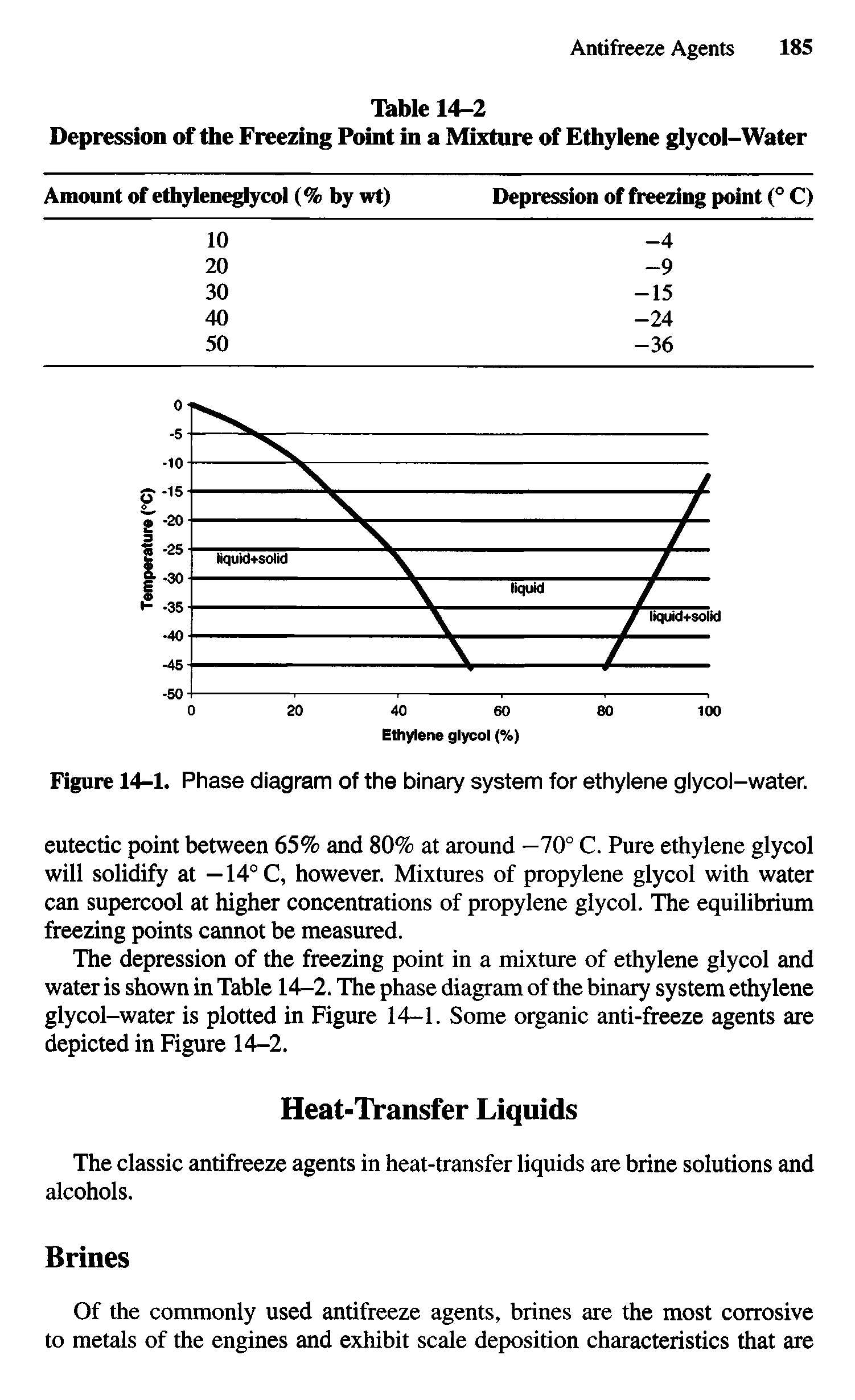
Ethylene glycol, phase diagram Big Chemical Encyclopedia
Ethylene Glycol 3BE0105 CymitQuimica
Ethylene Glycol Structural Formula
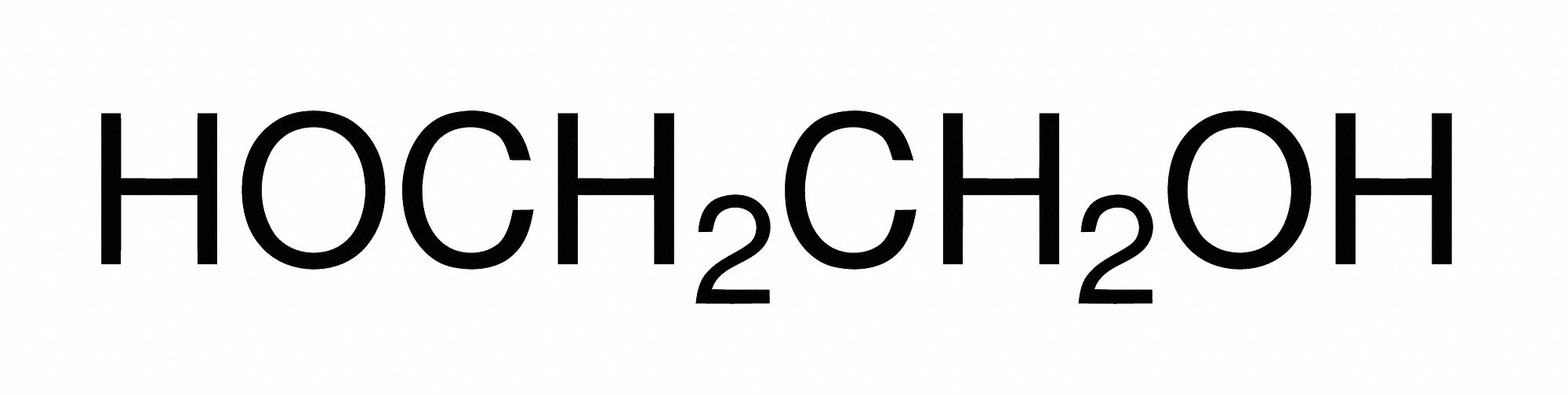
SIGMAALDRICH Ethylene Glycol, Molecular Weight 62.07 45ZY65PHR10461G Grainger

Solved Equal Volumes Of Ethylene Glycol Molar Mass And Water My XXX Hot Girl

Ethylene Glycol Density Chart Hot Sex Picture

C2h6o2 ethylene glycol molecule Royalty Free Vector Image
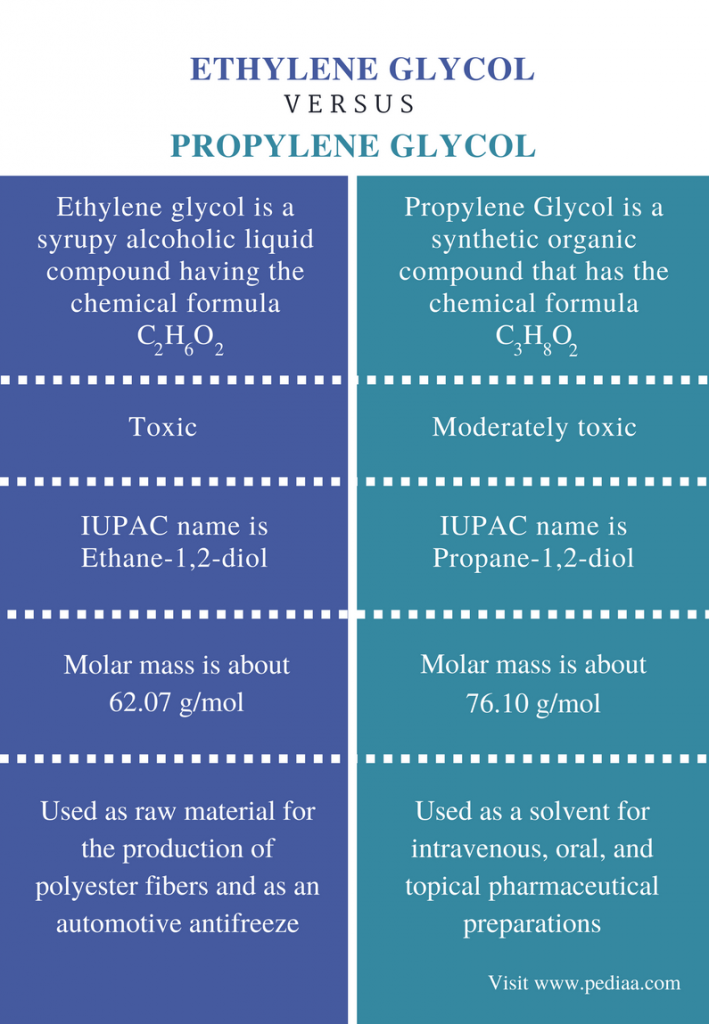
Difference Between Ethylene Glycol and Propylene Glycol Definition, Properties, Uses

Calculate the molarity of a solution containing 10.8g of ethylene glycol (C2H6O2) in 360 g of

Calculate the mole fraction of ethylene glycol (C2H6O2) in a solution containing 20
Finally, add together the total mass of each element to get the molar mass of C2H6O2: 24.0214 g/mol + 6.04764 g/mol + 31.9988 g/mol = 62.06784 g/mol. 5. Find Mole Fraction. To find the mole fraction and percentage of each element in C2H6O2, divide each total from step 3 by the total molar mass found in step 4:. Monograph ID M5122 Title Ethylene Glycol UNII FC72KVT52F Molecular formula C 2 H 6 O 2 Molecular weight 62.07 Percent composition C 38.70%, H 9.74%, O 51.55% Standard InChI