A solution of a strong alkali at concentration 1 M (1 mol/L) has a pH of 14. Thus, in most problems that arise pH values lie mostly in the range 0 to 14, though negative pH values and values above 14 are entirely possible. Weak acid/base. Weak acids/bases only partially dissociate in water. Finding the pH of a weak acid is a bit more complicated.. We can calculate the pH of a buffer using the Henderson-Hasselbalch equation. pH = pKa + log[A −] [HA] Before the equivalence point the concentration of unreacted acetic acid is. [CH3COOH] = (mol CH3COOH)initial − (mol NaOH)added total volume = MaVa − MbVb Va + Vb. and the concentration of acetate is.

PH DE HIDRÓXIDO DE SODIO AÑADIENDO GRAMOS DE NaOH CAMBIANDO LA CONCENTRACIÓN DE LA SOLUCIÓN

AcidBase Titrations General Chemistry
Naoh
Naoh acid or base vmlomi

Calculate Ph of 105M of naoh Brainly.in
The Soultion of naoh contains 0.04 gm of naoh per litre. Its Ph is
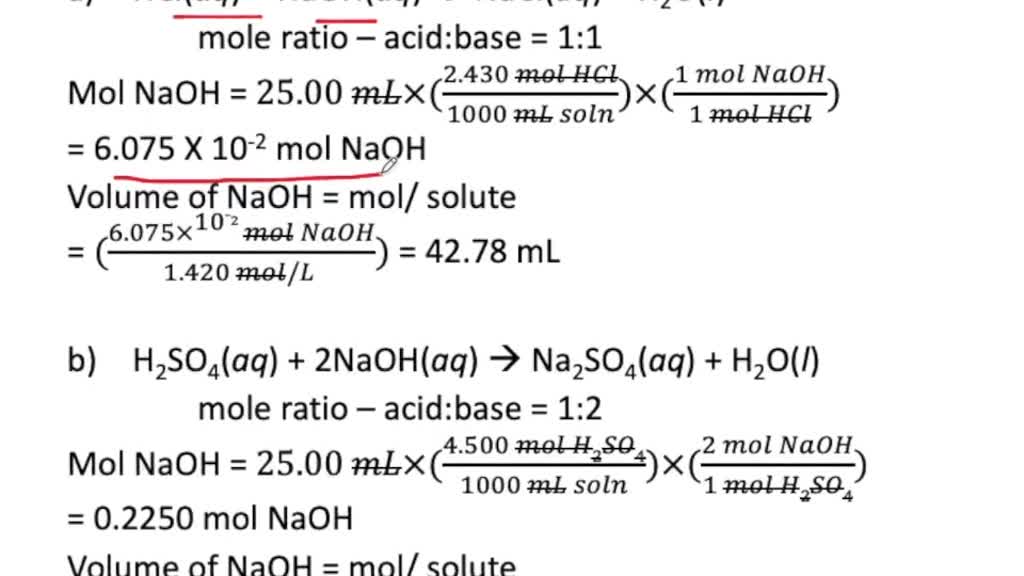
SOLVEDCalculate the volume in milliliters of a 1.420 M NaOH solution required to titrate the
21. Calculate the PH of a solution which contain 9.9 ml 1 M HCL and 100ml of 0.1 M NaOH

The pH of a solution obtained by mixing 100 ml of 0.2 M CH3COOH is with 100 ml of 0.2 M NaOH

A Amostra De 100 Ml De Naoh EDUCA
/prepare-sodium-hydroxide-or-naoh-solution-608150_FINAL-696b52d6f90b4b1383ec8f95db73a1f3.png)
How to Prepare a Sodium Hydroxide or NaOH Solution

Find the pH of a Buffer after adding NaOH YouTube
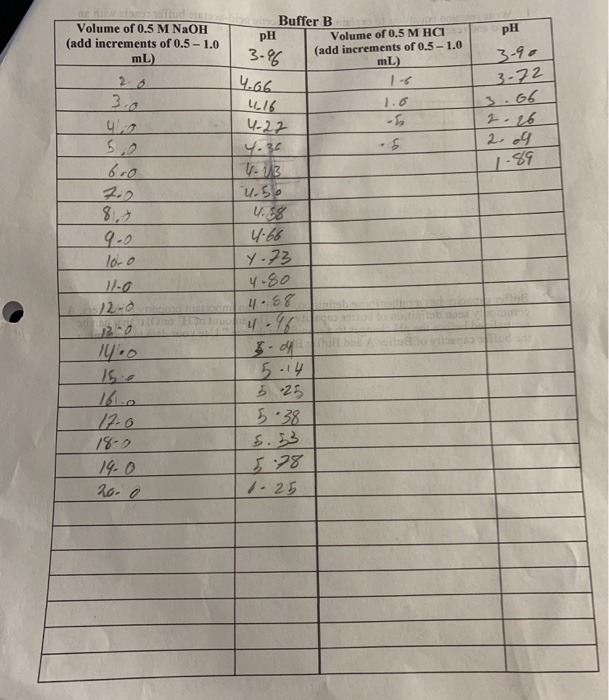
Solved pH Volume of 0.5 M NaOH (add increments of 0.5
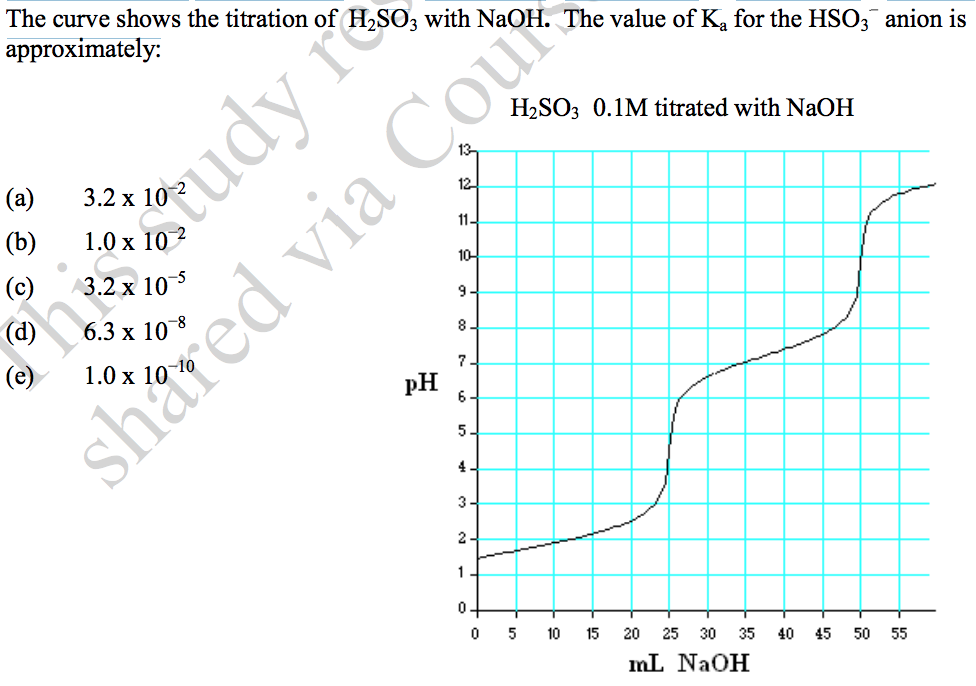
Solved The curve shows the titration of H2SO3 with NaOH. The

Solved 3. Calculate the pH of a 0.0010 M NaOH solution and
The pH of a solution obtained by mixing 100ml of 0.2 M CH3COOH with 100 ml of 0.2 M NaOH will be
Calculate pH of 0.01M solutions of NaOH

Acids Facts, Summary, Weak & Strong ALevel Chemistry Revision

Calculate The Ph Of The Following 0 5 M M Hcl If Chegg Hot Sex Picture

How To Calculate Ph From Molarity Of Naoh
NaOH is a strong base, so this will produce 0.1mol/L of OH ions in solution. This will produce a pH of 13. You will need to take the negative log of 0.1 to find the pOH. This will work out to be 1. Since pH + pOH = 14. We can calculate the pH to be 13. This assumption we made about the base can only be used for strong bases which dissociate.. Q. Calculate the pH at the equivalence point during the titration of 0.1 M, 25 ml CH3 COOH with 0.05 M NaOH solution ka (CH3 COOH)=1.8×10−5. Q. 20 cm3 of x M solution of HCl is exactly neutralised by 40 cm3 of 0.05 M N aOH solution, the pH of HCl solution is: Q. Calculate the pH at the equivalence point of the titration between 0.1 M CH3COOH.